Eliminate 3D bio-printing blockages
The technology
Gelatin methacryloyl (GelMA) hydrogels demonstrate a well-recognized cytocompatible behavior [1] and therefore, are ideal when developing bio-inks for engineering connective, epithelial, muscle, and nervous tissues. However, high printing pressures and ultimately nozzle blockages are recurrent issues when bioprinting with GelMA. Not only bioink losses is a hurdle due to blockages but printing within a high-pressure range is known to negatively impact post-printing cell viability in the 3D constructs. The temperature and time-dependent viscoelastic behavior of GelMA has a direct effect on its printability performance in extrusion-based systems.
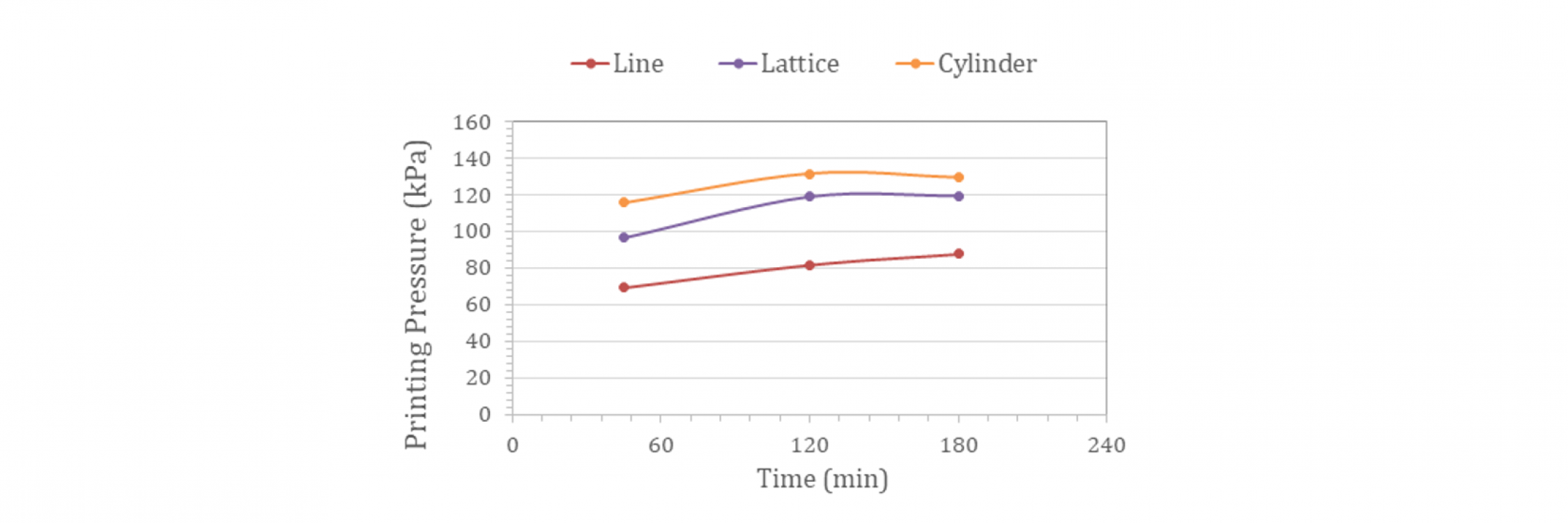
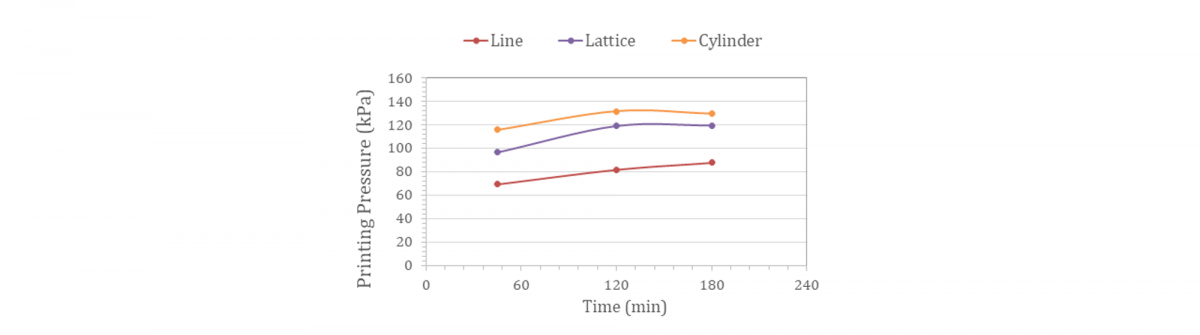
Claro™ developed ClaroBGI800, a gelatin-based biomaterial that combines all the advantages of GelMA with previously unseen levels of flow. The rheological profile of ClaroBGI800 allows bioprinting at ambient conditions (21 ± 1°C) and without temperature control of the print-head. As an example, a hydrogel prepared with 10% w/w ClaroBGI800 is printable (no nozzle blockage) with a slight increment in printing pressure over time. Around 70 kPa is required to print a line using a 27G nozzle after 45 min of equilibration time inside the nozzle (rest condition). An increase to 88 kPa is observed after 180 min. A similar upward trend is observed when lattice (2 layers) and cylinder (6 layers) structures are printed. The printing pressure of lattice and cylinder geometries are not significantly different, ranging from 100-120 to 120-130 kPa after, respectively, 45- and 180-min printing time.
The demonstrated extrusion pressures for ClaroBGI800 indicate that the product is suitable for cell bio-printing, and confirm it has the potential to encourage long-term cell viability. Higher temperatures combined with a smaller nozzle aperture are recommended to achieve high-resolution printing of cell-laden structures.
The propriety Claro™ technology enables printing to be done with lower pressure and excellent shape fidelity, without affecting the bio-compatibility and final gel strength of the construct.
Reference
1. Wang Z, Tian Z, Menard F, Kim K. Comparative study of gelatin methacrylate hydrogels from different sources for biofabrication applications. Biofabrication. 2017;9(4):44101. doi:10.1088/1758-5090/aa83cf